Proteasomal control of division in Archaea
In eukaryotes, proteasome-mediated degradation of cell cycle factors triggers mitotic exit, DNA segregation, and cytokinesis, a process that culminates in abscission dependent on the protein ESCRT-III. By studying cell division in an archaeal relative of eukaryotes, Tarrason Risa et al. identified a role for the proteasome in triggering cytokinesis by an archaeal ESCRT-III homolog. Cell division in this archaeon was driven by stepwise remodeling of a composite ESCRT-III–based division ring, where rapid proteasome-mediated degradation of one ESCRT-III subunit triggered the constriction of the remaining ESCRT-III–based copolymer. These data strengthen the case for the eukaryotic cell division machinery having its origins in Archaea.
Science, this issue p. eaaz2532
Structured Abstract
INTRODUCTION
Eukaryotes likely arose from a symbiotic partnership between an archaeal host and an alpha-proteobacterium, giving rise to the cell body and the mitochondria, respectively. Because of this, a number of proteins controlling key events in the eukaryotic cell division cycle have their origins in archaea. These include ESCRT-III proteins, which catalyze the final step of cytokinesis in many eukaryotes and in the archaeon Sulfolobus acidocaldarius. However, to date, no archaeon has been found that harbors homologs of cell cycle regulators, like cyclin-dependent kinases and cyclins, which order events in the cell cycle across all eukaryotes. Thus, it remains uncertain how key events in the archaeal cell cycle, including division, are regulated.
RATIONALE
An exception to this is the 20S proteasome, which is conserved between archaea and eukaryotes and which regulates the eukaryotic cell cycle through the degradation of cyclins. To explore the function of the 20S proteasome in the archaeon S. acidocaldarius, we determined its structure by crystallography and carried out in vitro biochemical analyses of its activity with and without inhibition. The impact of proteasome inhibition on cell division and cell cycle progression was examined in vivo by flow cytometry and super-resolution microscopy. Following up with mass spectrometry, we identified proteins degraded by the proteasome during division. Finally, we used molecular dynamics simulations to model the mechanics of this process.
RESULTS
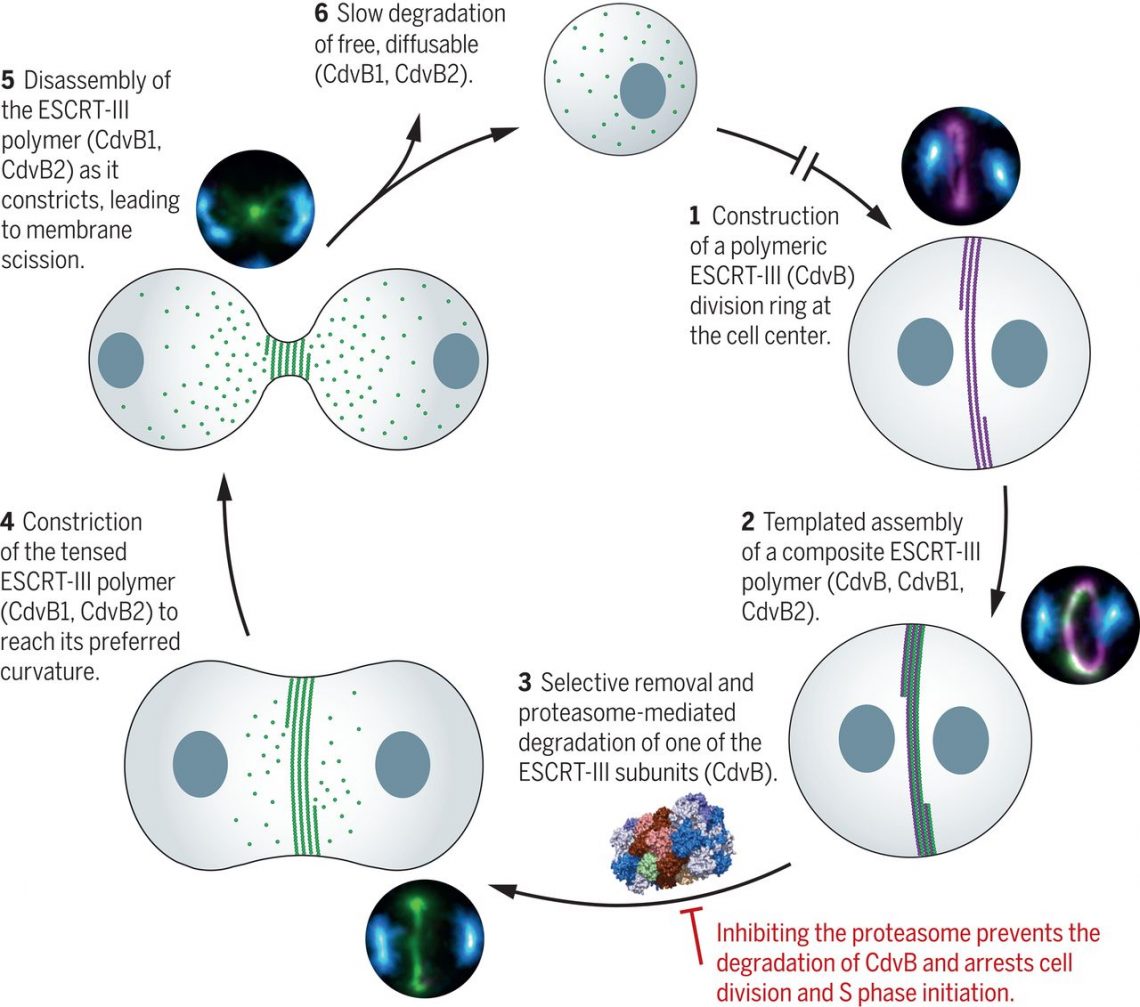
Here, we present a structure of the 20S proteasome of S. acidocaldarius to a resolution of 3.7 Å, which we used to model its sensitivity to the eukaryotic inhibitor bortezomib. When this inhibitor was added to synchronous cultures, it was found to arrest cells mid-division, with a stable ESCRT-III division ring positioned at the cell center between the two separated and prereplicative nucleoids. Proteomics was then used to identify a single archaeal ESCRT-III homolog, CdvB, as a key target of the proteasome that must be degraded to enable division to proceed.
Examining the localization patterns of CdvB and two other archaeal ESCRT-III homologs, CdvB1 and CdvB2, by flow cytometry and super-resolution microscopy revealed the sequence of events that leads to division. First, a CdvB ring is assembled. This CdvB ring then templates the assembly of the contractile ESCRT-III homologs, CdvB1 and CdvB2, to form a composite division ring. Cell division is then triggered by proteasome-mediated degradation of CdvB, which allows the CdvB1:CdvB2 copolymer to constrict, pulling the membrane with it. During constriction, the CdvB1:CdvB2 copolymer is disassembled, thus vacating the membrane neck to drive abscission, yielding two daughter cells with diffuse CdvB1 and CdvB2.
CONCLUSION
This study reveals a role for the proteasome in driving structural changes in a composite ESCRT-III copolymer, enabling the stepwise assembly, disassembly, and contraction of an ESCRT-III–based division ring. Although it is not yet clear how proteasomal inhibition prevents S. acidocaldarius cells from resetting the cell cycle to initiate the next S phase, these data strengthen the case for the eukaryotic cell cycle regulation having its origins in archaea.
The model shows the sequential stages of the division process in S. acidocaldarius (labeled 1 to 6), together with the corresponding stage-specific images. DNA is in blue, CdvB in purple, and CdvB1 and CdvB2 in green. The broken arrow represents an extended period where cells progress through G1, S, and G2. Note that Vps4 (not shown) is likely required for ESCRT-III polymer disassembly.
Abstract
Sulfolobus acidocaldarius is the closest experimentally tractable archaeal relative of eukaryotes and, despite lacking obvious cyclin-dependent kinase and cyclin homologs, has an ordered eukaryote-like cell cycle with distinct phases of DNA replication and division. Here, in exploring the mechanism of cell division in S. acidocaldarius, we identify a role for the archaeal proteasome in regulating the transition from the end of one cell cycle to the beginning of the next. Further, we identify the archaeal ESCRT-III homolog, CdvB, as a key target of the proteasome and show that its degradation triggers division by allowing constriction of the CdvB1:CdvB2 ESCRT-III division ring. These findings offer a minimal mechanism for ESCRT-III–mediated membrane remodeling and point to a conserved role for the proteasome in eukaryotic and archaeal cell cycle control.